Marketing moves at warp speed. For marketers and creative teams eager to set their campaigns free into the world, it can feel like compliance moves at a glacial pace.
However, marketing compliance is essential, especially in the pharmaceutical and healthcare industries. Failure to follow medical, legal, and regulatory (MLR) guidelines can land companies in major hot water. For example, if a pharmaceutical company releases a new medication and advertises the benefits without disclosing its side effects or risks, it could mislead consumers and potentially risk their health and safety.
Non-compliance may lead to lofty fines, legal liabilities, and irreparable damage to a company’s reputation. MLR review ensures all marketing materials adhere to strict regulatory standards and accurately represent products and treatments.
What we'll cover
Table of contents
What is medical, legal, and regulatory (MLR) compliance?
MLR compliance is the adherence to industry-specific standards and regulations that ensure all marketing and promotional materials for medical products are accurate, balanced, and include all necessary information about risks and benefits.
Whether marketing teams are creating a postcard or a press release, they must follow the regulatory compliance standards that govern heavily regulated industries. MLR compliance allows them to create impactful campaigns that resonate with target audiences and follow governance standards throughout the content and campaign lifecycle.
To fully understand MLR compliance, we also need to discuss MLR review. The MLR review process is a critical step in ensuring marketing compliance. Though the two terms are often used interchangeably, MLR review is the system of checks and balances an organization goes through to ensure campaigns adhere to MLR compliance standards.
Put simply: MLR review is the process, and MLR compliance is the objective.
Why is MLR compliance so important?
MLR compliance doesn’t exist to make marketers' lives difficult. It’s a safeguard to protect consumers and their information from deceptive or unfair practices. Regulatory bodies throughout the United States and abroad set and enforce the rules and regulations pharmaceutical and healthcare companies must follow.

This includes:
- The Food and Drug Administration (FDA): Regulates advertising and promoting prescription drugs, biologics, and medical devices in the U.S.
- The Federal Trade Commission (FTC): Enforces truth-in-advertising laws and regulates claims about drugs, devices, and healthcare products.
- The European Medicines Agency (EMA): Regulates medicines for humans and animals in the European Union (EU).
- PhRMA Code on Interactions with Healthcare Professionals: Provides guidelines for ethical interactions between pharmaceutical companies and healthcare professionals.
Let’s say a pharma company fails to follow MLR compliance guidelines. The company releases marketing materials that include potentially misleading information about a prescription drug. When the regulatory body finds out about the company’s non-compliance, it may send the company a warning letter or issue civil monetary penalties. Severe violations can even lead to criminal prosecution, permanently eroding consumer trust.
A similar scenario unfolded when pharmaceutical giant Novartis failed to include all safety risks for its heart drug Entresto. The Prescription Medicines Code of Practice Authority (PMCPA) in the U.K. ruled that Novartis violated multiple clauses of the code by not outlining the drug’s safety risks for liver and kidney damage.
MLR compliance is about more than avoiding negative repercussions. Companies that follow the rules and regulations can bring their products to market faster. By ensuring their marketing materials, product claims, and supporting documentation follow regulatory requirements, MLR teams minimize the need for revisions and additional information requests, accelerating their approval timelines.
There are also many long-term benefits. Companies with strong reputations for MLR compliance and ethical practices build trust. Regulatory bodies, healthcare professionals, and consumers view the company more favorably. This positive perception can facilitate smoother interactions and faster approvals.
Key MLR terminology to know
Marketing professionals who work with pharmaceutical, healthcare, or life sciences companies must be fluent in MLR terminology to navigate complex regulations. A common understanding of terminology also helps cross-functional teams of marketing, legal, regulatory affairs, and medical professionals communicate and streamline reviews and approvals.
Here are some common terms and acronyms you’ll want to know:
- Compliance: The process of adhering to the rules, regulations, laws, and ethical standards that govern a regulated industry. In the healthcare and pharmaceutical industries, this can include patient safety, patient privacy, and ethical practices.
- Regulatory affairs: The team of professionals responsible for ensuring compliance with the rules, regulations, laws, and standards.
- Adverse event (AE): In MLR compliance, an adverse event refers to any undesirable occurrence or experience a research participant has during a clinical trial or study involving a pharmaceutical product or medical device.
- Labeling: The set of written, printed, or graphic information that appears on pharmaceutical products, medical devices, or packaging.
- Clinical trials: A crucial part of the drug development process, clinical trials are medical research studies that test the safety and efficacy of new drugs, medical devices, or other interventions before they can be approved for patient use.
- Off-label use: Refers to the practice of prescribing, dispensing, or administering a medication for use in a way not approved by the FDA.
- Investigational New Drug (IND): A step in the drug development process where a drug must undergo rigorous MLR review to ensure compliance with regulations and patient safety.
- New Drug Application (NDA): The vehicle through which drug sponsors propose to the FDA that a new drug product be approved for marketing in the United States.
Breaking down each component of MLR compliance
MLR compliance consists of three components: medical, legal, and regulatory. Let’s take a closer look at each of these components.
Medical
This phase of the process involves validating marketing claims’ accuracy. Medical experts analyze scientific and clinical information presented in promotional and non-promotional materials. Their goal is to ensure the safety and efficacy of a pharmaceutical product.
Like detectives, medical experts gather valid, reliable scientific evidence to support all claims and communication. They must accurately present clinical data and information, and monitor adverse effects and other health-related issues to determine product safety.
Medical reviewers may include:
- Medical directors
- Medical writers
- Medical affairs professionals
- Pharmacists/PharmDs
- Physicians/MDs
A company may also outsource regulatory reviews to a consulting firm.
Legal
The legal aspect of MLR compliance protects pharmaceutical organizations from legal and ethical violations related to the marketing and promotion of drugs. Legal experts conduct a thorough review process to ensure that promotional and non-promotional materials comply with applicable laws, regulations, and industry-specific requirements.
These legal safeguards protect both consumers and drug makers. Legal experts ensure drug makers can exercise their intellectual property rights freely and that content doesn't violate existing trademarks, copyrights, or patents. They also verify compliance with privacy laws and regulations related to handling personal and sensitive information.
The legal review team may include:
- In-house legal counsel/attorneys
- Corporate counsel
- Legal managers
- Legal directors
Regulatory
Governing bodies, including the FDA, EMA, and the Federal Trade Commission (FTC), preserve regulatory standards and guidelines in the pharmaceutical industry. Their job is to verify that marketing practices are consistent with the terms of a product’s marketing authorization or product license. All labeling, promotional, and advertising practices must be truthful, straightforward, and compliant with regulatory standards.
Regulatory reviewers may include:
- Regulatory operations professionals
- Regulatory affairs specialists
- Regulatory affairs managers/directors
- Compliance specialists/officers
- Global labeling managers
Most important MLR compliance challenges

The MLR process can often create friction and frustration between involved parties. Though review guidelines are clear-cut, departments may not always have a clear roadmap to navigate the process.
These are a few of the most common challenges:
Navigating complex regulations
Pharmaceutical companies struggle to stay ahead of frequent regulation changes from the FDA and FTC. Ensuring compliance across different regions and markets comes with its own set of challenges. Companies must also accurately interpret regulatory standards and guidelines to avoid strict penalties and fines.
Maintaining accuracy and transparency
To preserve accuracy, you need accurate data and evidence. This data validates reports to governing bodies, and it can’t live in a black box — entities throughout the company should have visibility into the process. However, this isn’t the case in many organizations.
International compliance
For organizations with a global presence, it’s difficult to ensure compliance across borders. Compliance regulations vary across countries and regions. This makes developing consistent processes that align with the strictest regulatory requirements tough. Companies that don’t know how to navigate market-specific requirements like local disclaimers have extra hoops to jump through.
Resource constraints
It’s not always easy to find professionals who specialize in medical writing, regulatory affairs, and legal compliance. This is especially true for organizations with geographically diverse marketing teams. Personnel shortages can hinder an organization’s ability to employ and retain those who can conduct thorough MLR reviews across departments.
Best practices for MLR compliance

Despite the challenges involved with MLR compliance, there are clear actions they can take to facilitate a smooth review process:
Developing comprehensive training programs
Knowledge is power. Your marketing teams, content creators, and other relevant team members should have a firm understanding of MLR requirements. Offer training programs to keep them informed of changes in regulations and industry standards. Regular training ensures everyone understands the importance of compliance and how to implement it in their work.
Standardizing your review process
A standardized MLR process subjects all promotional materials to the same rigorous review process. Consistency minimizes the risk of non-compliance from variations in approach or oversight. Standardization might include creating a standard glossary of terminology, automating tasks to ensure proper routing, or establishing control gates with key milestones.
Documentation and record-keeping
Comprehensive documentation provides a clear audit trail and enables your organization to demonstrate compliance with MLR requirements. Companies with strict record-keeping policies can ensure they’re using the most up-to-date versions of marketing materials — including feedback and revisions — for promotion.
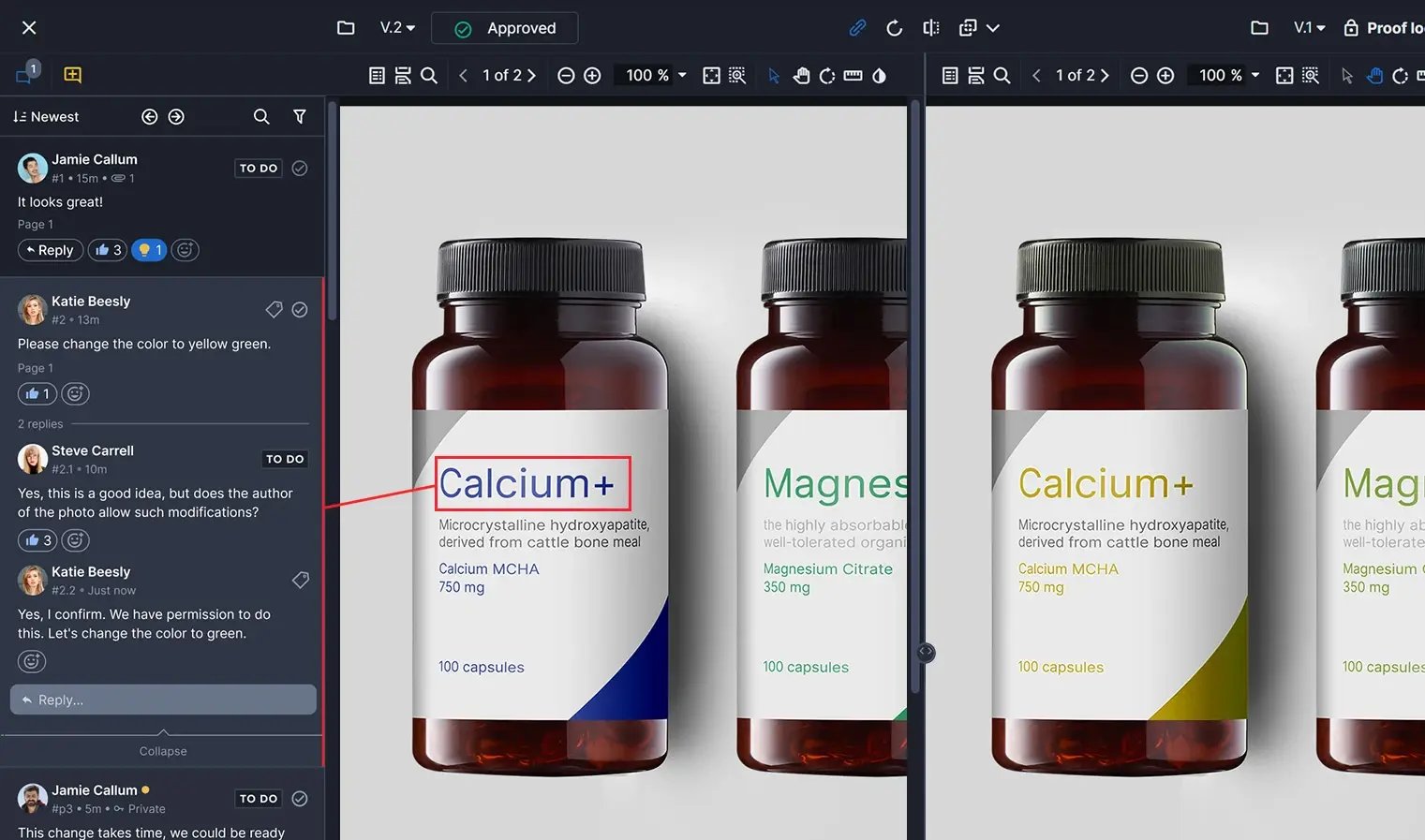
Leverage compliance management software
![]()
Using compliance management software can streamline the MLR review process and shave off significant time. It helps centralize documentation and ensures the most timely updates when regulations change. Compliance management software can also help teams:
- Build compliance-ready workflows to include the right decision-makers in the review process.
- Provide visibility into who made changes or approved assets in a workflow.
- Collaborate safely and securely with audited and validated internal controls so only authorized users can access documents.
Your organization can leverage many of its existing tools alongside compliance software to expedite the review process. Here are a few examples:
Content Management Systems (CMS) and Digital Asset Management (DAM) Tools
By integrating your CMS and DAM tools with your compliance management software, you can centralize file storage, keep your files organized, manage claims, and ensure everyone can access marketing assets. From one application, stakeholders can review content in real time, issue approvals, and keep track of multiple versions of a document. These are all critical tasks for MLR compliance.
Marketing Project Management Tools
Since cross-functional teams are often involved in the MLR review process, collaboration and communication are critical. Project management tools like Wrike and Asana help ensure effective project management, task assignment, resource allocation, and visibility.
Regular audits and assessments
To safeguard patient safety and mitigate a company’s legal and financial risks, audits and assessments are key. Your promotional materials, marketing activities, and related processes should be regularly reviewed to identify any compliance issues or potentially inaccurate claims. This is how companies build trust with healthcare professionals, patients, and the general public.
Achieve MLR compliance efficiently with Ziflow
Creative teams within the pharmaceutical, healthcare, and life sciences industries must understand MLR compliance and have a strong MLR review process. Failure to produce accurate and ethical marketing materials can lead to steep fines, serious penalties, and irrevocable damage to a company’s reputation.
Designed for creative teams working in highly regulated industries, Ziflow has the compliance features to move MLR reviews along more smoothly. It’s a leading collaborative proofing platform for regulatory and brand compliance.
Check out the Everyday Health Group uses Ziflow to manage their MLR process

With a track record that spans media giants like WarnerMedia, Viacom, and Google, Aaron's expertise shines through in multi-million dollar projects across various mediums, from traditional television to the dynamic realm of YouTube.





